Transition metal (TM) oxides are a fascinating class of materials, whose
properties can be suitably tuned
in a variety of ways; such as by selecting TM-ions/dopants having
preferred electronic configurations,
engineering the crystallographic site occupancy by dopants, controlling/modifying
the degree of
covalence of TM-O bonds, modifying lattice spacing(s), tuning phase
assemblage etc. Such modifications
done from the fundamental perspectives influence the performances of
TM-oxides for a variety of
applications, including their widespread usage as cathode-active materials in alkali
metal-ion batteries
(i.e., the Li-ion and Na-ion battery systems).
In the context of Li-ion batteries, ‘cation disordering’ that takes place at deep
states of delithiation of
Ni-containing ‘layered’ Li-TM-oxides leads to
structural-cum-electrochemical instability during charging
of Li-ion cells at potentials beyond 4.3 V (vs. Li/Li+). This forms a
major bottleneck towards raising the
operating voltage of Li-ion batteries and thus, leads to a compromise over the
energy density.
Nevertheless, our recent research results have indicated that optimal doping of such
‘layered’ Li-TM-
oxides with ion(s) having d10 electronic configuration (and, thus, no
OSPE) suppresses Ni-migration from
TM-layer to Li-layer (viz., ‘cation disordering’) and, thus, preserves
the structural integrity even upon
deep states of delithiation (i.e., at high cell voltages) [recently, published as;
Sharma et al., ACS Appl.
Mater. Interfaces 13[22] (2021) 25836].
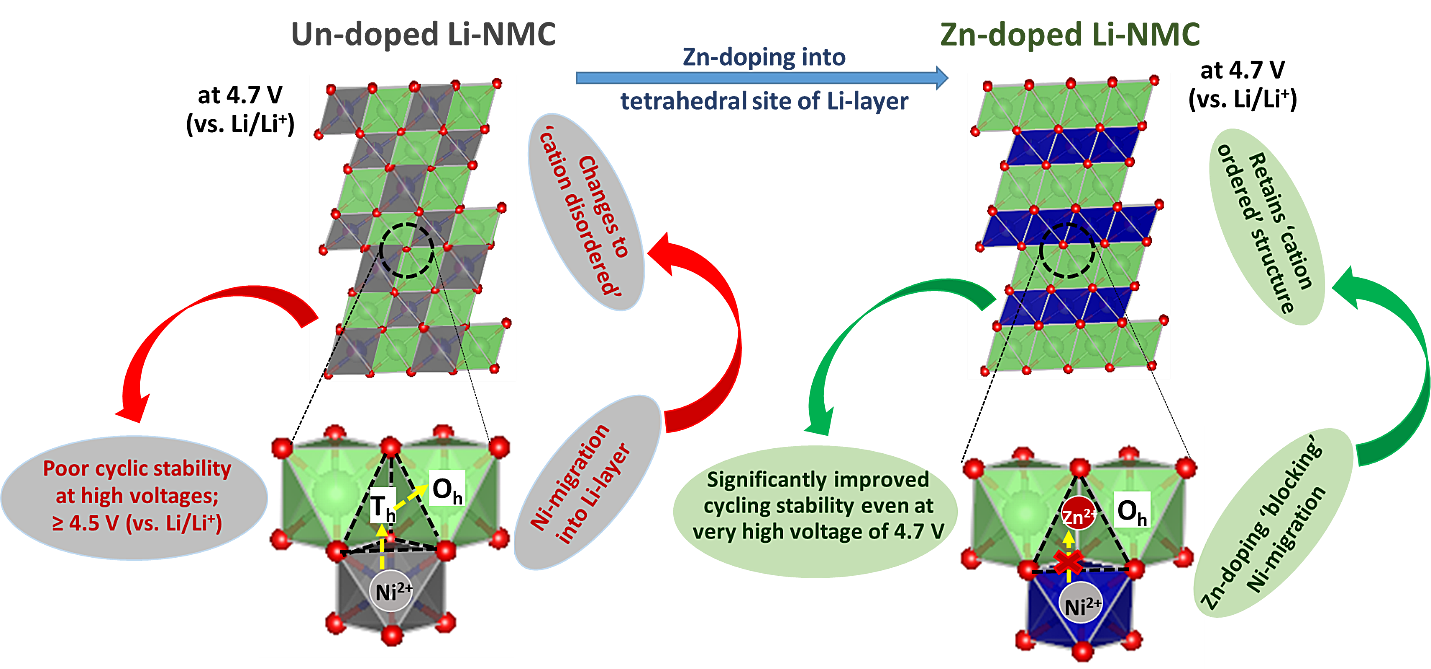
Sharma et al., ACS Appl. Mater. Interfaces 13[22] (2021) 25836
Figure: Effect of Tetrahedral site Zn-doping on the migration passage of Ni-containing Layered Transition Metal (TM) oxide on High Voltage Structural and Electrochemical Stability.In the context of the upcoming Na-ion battery system, O3-type ‘layered’ Na-TM-oxides are promising as cathode-active materials due to their inherently high initial Na-content (as compared to the P2 counterparts); but suffer from instabilities caused due to multiple phase transformations during Na- removal/insertion and sensitivity to air/moisture. We have been able to tune the composition and structural features to suppress the phase transitions upon Na-removal/insertion and also improve the air/water-stability in significant terms; so much so that long-term cyclic stability has been achieved with health/environment-friendly ‘aqueous processed’ electrodes (sans, usage of toxic/hazardous/expensive chemicals like NMP and PVDF). The changes in structural features, which have led to such outstanding water-stability, include differential contraction/dilation of the Na-‘inter-slab’/TM-‘slab’ spacing and partial occupancy of the dopant at tetrahedral sites of the structure [recently, published as; Kumar et al.; J. Mater. Chem. A 8 (2020) 18064].
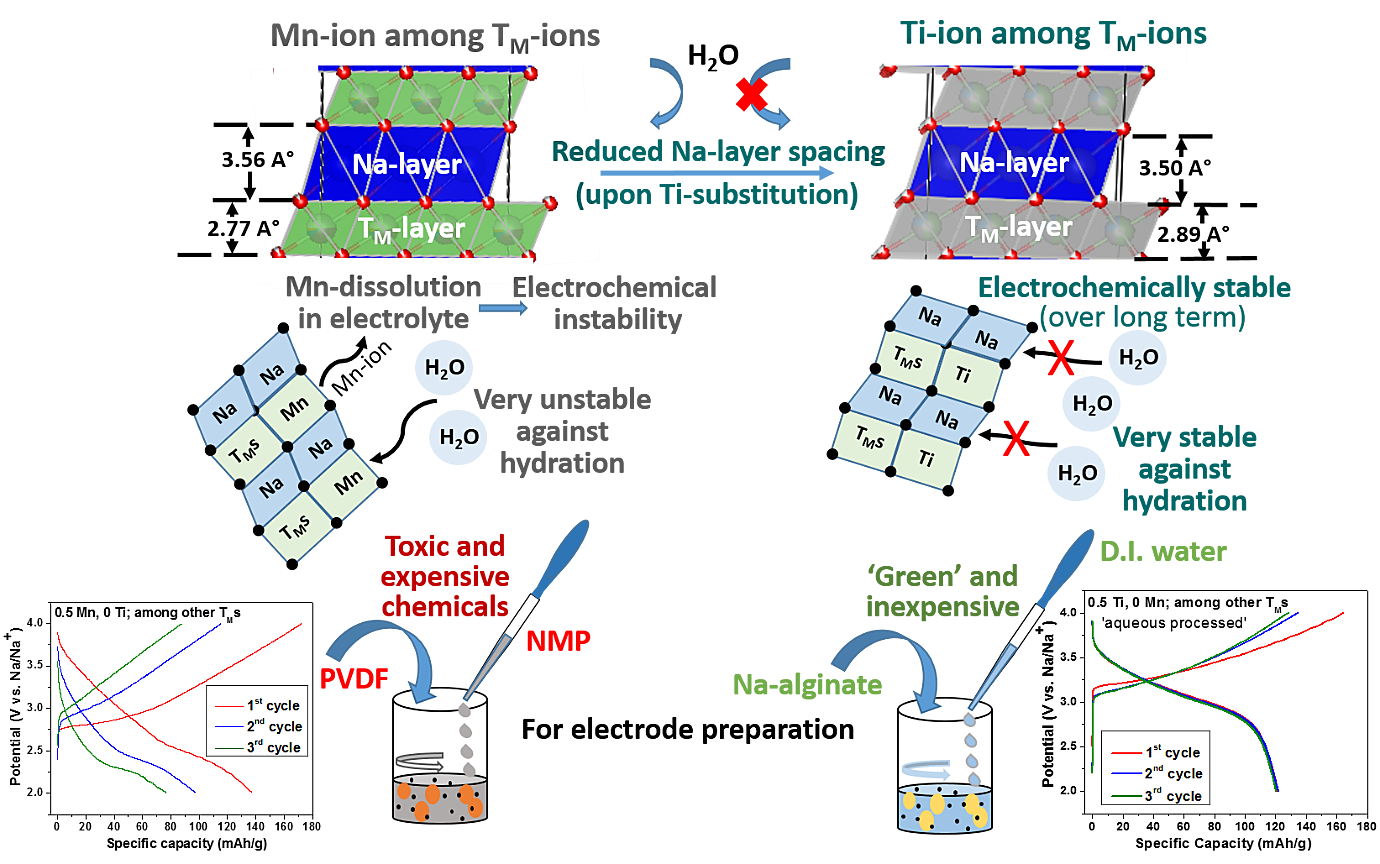
SKumar et al.; J. Mater. Chem. A 8 (2020) 18064
Figure: Tuning TM-ion ‘slab thickness’ and alkali metal-ion ‘inter-slab spacing’ of Na-TM-oxides in opposite terms (and also suppressing TM-dissolution in electrolyte) leads to outstanding air/water-stability and long-term electrochemical stability.People Involved -