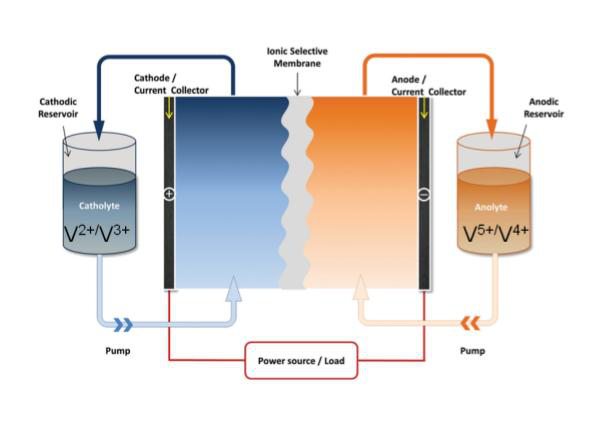
Large-scale energy storage systems based on vanadium redox flow batteries (VRFBs)
are developed for
the deployment of renewable energy technologies. VRFB is easy to scale and the
energy and power can
be decoupled. The materials used for the electrode have a crucial role on the
performance of VRFB. It is
reported that the pre-treatment of the electrode increases the hydrophilicity and
creates functional
groups (carbonyl, carboxyl and hydroxyl) on the electrode surface, thereby
influencing the kinetics. The
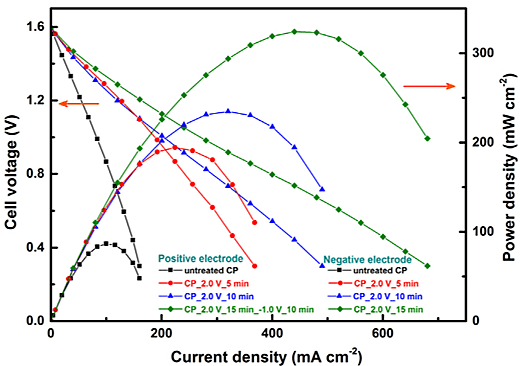
figure below presents the performance of VRFB with untreated and electrochemically
treated Toray
carbon papers (CPs). The electrochemically generated functional groups on CP can be
confirmed by XPS.
It is found that the asymmetrically configured cell with CP_2.0 V_15 min_-1.0 V_10
min at the positive
electrode and CP_2.0 V_15 min at the negative electrode offers maximum power density
of ~325 mW
cm-2, and it is much higher than that of symmetric cell using untreated
CP electrodes (~85 mW cm-2).
[Note: CP_2.0 V_15 min_-1.0 V_10 min indicates CP electrode after the initial
treatment at
+2.0 V for 15 min treated subsequently at -1.0 V for 10 min].