


Silicon and silicon-based composite material have the potential as next-generation anode material for lithium-ion batteries due to its higher specific capacity (~ 4200 mAh/g, Li4.4Si), low cost, low working voltage, and environmental benignity than conventional graphite (~ 350 mAh/g). Hence, it can aid to achieve a driving range of 300 miles for electric vehicles. NCPRE battery research group has already synthesized Si from sand (SiO2) by microwave heating, which is a fast and energy-efficient approach (Figure). However, Si as anode has some limitations, such as first cycle capacity loss and very high-volume expansion (~ 300-400%) during continuous charging and discharging process. Si nanoparticle, nano-rods, and nanowire are the possible solutions to reduce volume expansion up to a specific limit, but nano-Si is highly reactive and can dissolute within electrolyte during cycling.
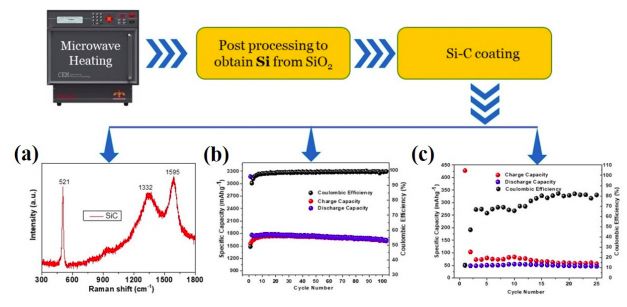
Therefore, mixing of Si with carbon is another approach to reduce the volume expansion problem because carbon can accommodate the volume expansion and provides the electrical conductivity chain from bulk to current collector. The team is currently working on the development of SiC || Ni-rich Li-NMC811 full cell lithium-ion battery to achieve 300Wh/kg energy density at the cell level for electric vehicles. However, this chemistry has some challenges, such as first cycle capacity loss and capacity fading during cycling. The target (300Wh/kg) can be achieved with optimization in higher cut-off voltage (≥4.4V), active material loading (porosity), and cathode/anode modifications. The figure shows the synthesis process for SiC anode and characterization results for SiC half-cell and SiC||Li-NMC811 full cell. Figure (c) represents the capacity and cyclic performance of a full cell utilising Si-C anode and NMC811 cathode on a 2016-coin cell. The optimization to this material with currently available high-performance cathodes would result in a more efficient and high energy density battery.
