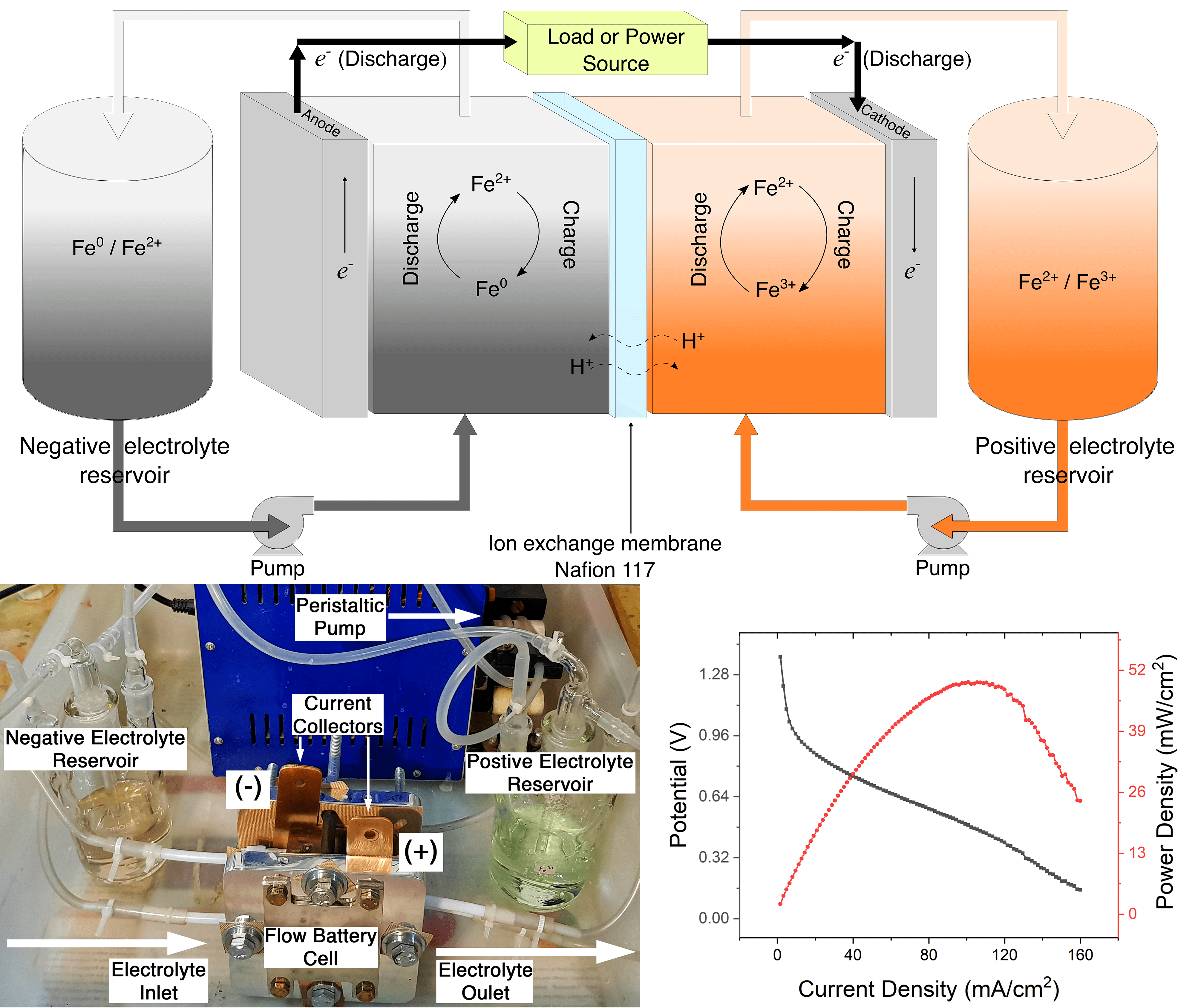
A subset of immense battery field technology is the Redox Flow Batteries (RFB). Unlike normal batteries where the reactants are usually solid, a redox flow battery uses liquid reactants, which can be stored in large tanks. This decouples energy and power, and allows a modular design with flexible operation. A rather benign and cheaper type is the all iron Redox Flow Battery.
An all-iron Redox Flow Battery utilizes reaction among iron metal and two other soluble iron compounds in two different oxidation states to store and discharge electricity. Of course, the compounds are separated by a membrane to prevent spontaneous reactions. Whenever electric power is required, the reactants flow, and a chemical reaction is started at the electrodes. The exhausted chemicals then can be regenerated when power is in excess by reversing the reaction as well as the flow. A typical module generates a voltage of ~1.0 V and the current is proportional to the size of the electrode. One such device is made by one of the NCPRE teams (M. Shariq Anwar under the guidance of Prof. Arindam Sarkar) in Energy Storage Group along with its power output characteristics. Of course, the power generated by each module is low compared to other RFBs, the great advantage of using iron is its availability in our country and low toxicity. One can, thus, envision a giant battery made by assembling several thousand modules capable of storing and discharging several 100 MWh of energy.